I. BOP EFFORTS TO REDUCE PRESCRIPTION MEDICATION COSTS
- The BOP has not correctly assessed the budgetary impact of its planned initiatives to reduce increasing costs for prescription medications. As a result, future initiatives may result in increased rather than decreased costs. In our judgment, the estimated costs and savings of the BOP's proposal to move to a Central Fill pharmacy are inaccurate. The BOP estimated that when Central Fill is fully implemented it will save $1.14 million annually; however, we found that Central Fill may actually increase costs by approximately $900,000 annually. We also found that the BOP wastes over 5 percent of its prescription medication costs due to inmate transfers, confiscations, expiration of medication, and other reasons. We estimated the waste to be approximately $2.81 million in FY 2004. Finally, we found that the BOP's OTC policy has not been consistently applied and implemented across all BOP institutions.
As noted in the Background section of this report, the BOP is concerned about the increasing cost of prescription medications, the difficulty in recruiting trained professionals, and the safety of administering increasingly complex medication regiments. To reduce costs and address other concerns, the BOP has proposed several changes to its pharmacy services. These include the planned realignment of institutions based on levels of care, Central Fill, Central Processing, electronic medical records system, and an OTC policy.
Central Fill Cost-Benefit Analysis
Through the BOP's Central Fill proposal, pharmacists at BOP institutions would review prescriptions for contraindications and then transmit the prescriptions electronically to the VA Centralized Mail Outpatient Pharmacy Center in Dallas, Texas. The VA would fill the prescriptions and mail them overnight to the institution.
In March 2004, the BOP completed a cost-benefit analysis of its Central Fill proposal to estimate the impact on the BOP's prescription medication costs. Initially, the BOP developed three different estimates of savings, ranging from $1.14 to $6.42 million annually, as shown in Figure 9.
| FIGURE 9. BOP's CENTRAL FILL COST SAVINGS ESTIMATES |
|
| Source: BOP analysis |
The difference in savings for each estimate is based on whether the BOP can access pricing related to Pub. L. No. 102-585 (1993), which provides a discount known as the Federal Price Ceiling (FPC) on brand name prescription medications for which there are no generic equivalents. This discount was granted to the four federal agencies that are the largest purchasers of prescription medications: the VA, the Department of Defense, the Department of Health and Human Services, and the Coast Guard. As a result, the FPC discount is commonly known as “Big 4” pricing. Based on a legal opinion provided by the VA, it is a violation of Pub. L. No. 102-585 (1993) for an agency to pass on its Big 4 pricing to any other entity; therefore, Big 4 pricing is not available to the BOP.
As shown in Figure 9, the BOP Workgroup calculated the three estimates based on different assumptions of prescription medication prices charged by the VA.
- Estimate 1 – The net projected savings of $6.42 million is based on the BOP's ability to buy all prescription medications from the VA using Big 4 pricing. However, the estimated savings of $6.42 million is not attainable since Big 4 pricing is not available to the BOP.
- Estimate 2 – The net projected savings of $4.12 million is based on restocking the VA facility for Big 4 prescription medications purchased through the Department of Health and Human Services Supply Services Center, located in Perry Point, Maryland. During the audit, we determined that the Supply Services Center was passing on its Big 4 savings to the BOP, in violation of Pub. L. No. 102-585 (1993). As a result, the Supply Services Center has discontinued this practice. Therefore, the estimated savings of $4.12 million also is not attainable.
- Estimate 3 – The net projected savings of $1.14 million is based on the BOP purchasing prescription medications through the FSS at non Big 4 prices to restock the VA facility for Big 4 prescription medications. This process could avoid any legal issues related to the BOP receiving Big 4 pricing on prescription medications, and results in the only feasible estimate. However, as described below, we believe that this estimate is also not accurate.
According to BOP officials, they are planning to request that Congress amend Pub. L. No. 102 585 (1993) to make the BOP eligible for Big 4 pricing. Given the potential for savings by the BOP in its prescription medication costs, we believe this is an important strategy, and we recommend that the BOP aggressively pursue this effort. However, at the time of our audit, Estimate 3 appeared to be the only feasible estimate. Consequently, we based our analysis on it.
The BOP based its net savings of $1.14 million for Estimate 3 on estimated gross savings of $7.74 million, minus estimated costs of $6.6 million. The $7.74 million estimated gross savings consists of $4.94 million from gross purchase savings, $2.39 million from the reduction of waste, and $0.41 million from labels and vials that will no longer be used. The estimated $6.6 million cost consists of $5.6 million in VA fees, $1 million in shipping fees, and $4,000 in information technology. A detailed breakdown of how the BOP reached Estimate 3 is shown in Figure 10.
| FIGURE 10. BOP COST-BENEFIT ANALYSIS |
|
| Source: BOP analysis |
We reviewed the BOP's cost-benefit analysis for Estimate 3 and determined that the savings related to the Central Fill proposal are overstated. As shown in Figure 10, the BOP estimated that a fully implemented Central Fill would save $1.14 million per year. However, our analysis shows that Central Fill could actually cost the BOP as much as approximately $900,000 per year.
Analysis of Gross Purchase Savings
As previously stated, the BOP estimated a savings of $4.94 million on prescription medications purchased through the VA by using Central Fill. The estimated-gross-purchase savings were based on a BOP survey of six institutions over a 1-year period.25 The BOP compared the costs of prescription medications purchased by the six institutions to prices paid by the VA for the same medication. Based on this analysis, the BOP calculated that Central Fill would save the six institutions $0.33 million, or 10.36 percent of their total prescription medication costs per year. The BOP then projected the 10.36 percent savings to the total BOP prescription medication costs for the same time period. Accordingly, the BOP calculated savings for all institutions at an estimated $4.94 million (10.36 percent of $47.71 million).
However, based on our analysis of the BOP's estimate, we found several errors and incorrect assumptions that resulted in gross purchase savings being overstated by $2.97 million. Specifically, we found that the: (1) data used to calculate the 10.36 percent savings, from the analysis of the six institutions, included two errors resulting in an overstatement of savings; (2) the savings estimate incorrectly assumed 100-percent usage of Central Fill by all BOP institutions; (3) analysis of the six institutions did not include all prescription medications purchased during the 1-year period; and (4) analysis compared the BOP and VA prices for different time periods.
Specifically, we found that the data used by the BOP to calculate the 10.36 percent estimated gross purchase savings included an error related to Ribavirin, a Hepatitis C medication, which resulted in an 1,803-percent difference. The BOP calculated savings for Ribavirin by using costs of $0.22 million for the six institutions divided by an estimated 17,486 pills purchased. This resulted in an estimated average price per pill for Ribavirin of $12.45. However, based on our analysis the number of pills purchased was actually 51,086, a difference of 33,600 pills. Using the adjusted number of pills, we estimated the average price of Ribavirin to be $4.26 per pill, a difference of $8.19, as shown in Figure 11.
| FIGURE 11. DETAIL ON IMPACT OF RIBAVIRIN ERROR |
|
| Source: BOP and OIG analysis of data provided by the BOP and the VA |
Based on our estimate, the percentage gross purchase savings for the six institutions is reduced from 10.36 percent to 6 percent. When we project the new percentage savings to all BOP institutions, we estimate gross purchase savings to be $2.86 million, compared to the original BOP calculation of $4.94 million. We brought this error to the attention of BOP management, who concurred with the OIG calculation and the recommended adjustment.
We also found that the BOP gross purchase savings estimate included two prescription medications, Videx® 200 milligrams (mg) chewable and Sular® 20 mg tablets, for which a VA price was not available for comparison. Instead of excluding these prescription medications from the gross purchase savings analysis, the BOP included $14,558 as a cost savings for the six institutions. Based on our analysis, the savings of the six institutions should be reduced by 0.46 percent, resulting in a 5.54 total estimated percent savings instead of 6 percent.
| FIGURE 12. DETAIL ON IMPACT OF VIDEX® AND SULAR® ERROR |
|
| Source: OIG analysis of data provided by the BOP and the VA |
As shown in Figure 12, we used the adjusted percent to project gross purchased savings to all institutions, and calculated the savings to be $2.64 million (5.54 percent x $47.72 million), rather than the BOP's estimate of $4.94 million, a difference of $2.3 million.
We also found that the BOP based its estimate of gross purchase savings on the assumption that all prescription purchases would occur through Central Fill. The BOP used the total prescription costs for all institutions of approximately $47.72 million during the 1-year period of its analysis to calculate estimated savings. However, the BOP expects that medical centers and FDCs will only purchase 50 percent of their prescription medications through Central Fill. We also noted that the Oklahoma FTC will not use Central Fill, and all other institutions will use Central Fill for 95 percent of their prescription medications. As a result, the BOP will use Central Fill to purchase only 74.5 percent of its total prescription medications.26 We applied the adjusted usage of 74.5 percent to the prescription medication costs used in the BOP's analysis, and found the total cost affected by Central Fill is reduced to $35.55 million, a difference of $12.17 million, as shown in Figure 13.
| FIGURE 13. ADJUSTMENT TO TOTAL COST ESTIMATE |
|
| Source: OIG analysis of data provided by the BOP and the VA |
This adjustment further reduces the BOP's estimated gross purchase savings to $1.97 million, a difference of $2.97 million from the BOP's original estimate of $4.94 million.
Finally, we found that the BOP did not include all prescription medications in its analysis of gross purchase savings for the six institutions. Instead, the BOP estimated the savings on just tablets and capsules, and excluded any other types of prescription medications. According to BOP officials, liquids and ointments were excluded to simplify the calculation. However, without estimating the costs or savings for all medications, the BOP could be overstating or understating the estimated total gross purchase savings. In addition, the time periods used for BOP and VA prices are not consistent. BOP prices were derived using the average price paid by the six institutions over a 1-year period from March 2003 through February 2004. The VA prices were based on a specific date during the time of the analysis. As a result, some BOP estimated gross purchase savings may be the result of timing differences in prescription prices.
The BOP's Central Fill proposal estimated savings of $2.39 million from the reduction of waste of prescription medications. The BOP based this savings on informal discussions with several BOP pharmacists, who indicated that the BOP could save about 5 percent of its total prescription medication costs through reduced waste. The 5 percent savings relies on the assumption that Central Fill will provide better inventory management, by preventing expiration of prescription medications and improving the ordering of crash cart medications.27
During our audit, we found many different causes of prescription medication waste, such as expired medicines, transfers of inmates, and confiscations. For example, the BOP does not ensure that prescription medications are transferred with inmates when they are moved to new facilities. Waste from confiscations is also caused by correctional officers seizing prescription medications during searches of inmates' cells. Central Fill would only reduce waste associated with expired prescription medications. With Central Fill, most institutions only maintain an inventory of emergency prescription medications, such as antibiotics and pain relievers, which, in theory, reduce waste caused by medications that expire before they are used.
Based on our analysis, the BOP overstated the estimated savings of $2.39 million due to the reduction of waste. We conducted a survey of all BOP pharmacists and had a response rate of 84 percent (106 responses out of 126 questionnaires sent). The results of our survey found that BOP pharmacists estimated that only 1.21 percent of waste is associated with expired medications rather than the 5 percent figure used by the BOP in its calculation.
| FIGURE 14. ADJUSTMENT TO WASTE ESTIMATE |
|
| Source: BOP and OIG analysis of costs and percentage of estimated waste |
As shown in Figure 14, we estimate savings from Central Fill related to a reduction in waste of $0.58 million (1.21 percent x $47.72 million), a difference of $1.81 million from the BOP's estimate.28 In addition, the BOP's analysis does not include the partial refunds that many BOP institutions receive when they return expired prescription medications, which would further reduce the estimate savings.
The BOP estimated that by switching to Central Fill it would reduce vial and label purchases and save an estimated $0.41 million per year. The BOP based this estimate on the assumption that 5 million prescriptions were filled during a 1-year period, requiring 2.75 million vials for 55 percent of prescriptions, and 5.5 million labels for 110 percent of prescriptions.29 However, from data we gathered for FY 2003 and FY 2004, the BOP only dispensed an average of 3.33 million prescriptions per year during the period. Therefore, based on our analysis, we estimated that the BOP will only use Central Fill for 83.4 percent or 2.78 million prescriptions.30 Using the same percentages for vials and labels estimated by the BOP, the number of vials used is reduced to 1.53 million and the number of labels used is reduced to 3.06 million, as shown in Figure 15.
| FIGURE 15. ADJUSTMENT TO VIALS AND LABELS SAVINGS ESTIMATE |
|
| Source: BOP and OIG analysis of number of prescriptions, vials, and labels |
As shown in Figure 15, we estimate that the BOP would save $229,231 in vials and labels by using Central Fill rather than its estimate of $412,500.
The BOP estimated that it will spend $6.6 million annually related to Central Fill. Of this total amount, $5.6 million would come from VA fees, $1 million from shipping, and $4,000 from information technology. Our analysis revealed that the BOP overstated prescription fees and shipping costs.
The BOP estimated that the fees paid to the VA for filling prescriptions would average $5.6 million annually. We found that this estimate is based on an assumed fee of $1.12 per prescription that the VA will charge to fill an estimated 5 million prescriptions per year. Because we determined that the BOP will more likely use the Central Fill for about 2.78 million prescriptions per year, the cost associated with VA fees would be $3.11 million as opposed to $5.6 million, shown in Figure 16.
| FIGURE 16. ADJUSTMENT TO PRESCRIPTION FEE COST |
|
| Source: BOP and OIG analysis of the number of prescriptions using Central Fill |
The BOP's estimated cost also included a $1 million charge for annual prescription shipping costs from the VA facility in Dallas, Texas, to 100 BOP institutions. The BOP estimated shipping cost is based on an assumption of 200 prescriptions per day, per institution, for 250 shipping days per year. This results in a total of 5 million prescriptions shipped per year. However, as stated previously, we estimated that the BOP will only use the Central Fill for about 3 million prescriptions per year. As a result, the number of prescriptions shipped per day, per institution, is reduced from 200 to 111, resulting in a $0.45 million reduction in the total shipping cost, as shown in Figure 17.
| FIGURE 17. ADJUSTMENT TO SHIPPING COST |
|
| Source: BOP and OIG analysis of costs related to shipping |
Summary of BOP and OIG Central Fill Cost-Benefit Analysis
During our review of the BOP's cost-benefit analysis for its Central Fill proposal, we found several errors and incorrect assumptions concerning the accuracy of the BOP's estimated savings. The BOP estimated savings of $1.14 million annually, which based on our analysis, is overstated by as much as $2.03 million. As a result, Central Fill may cost the BOP as much as $895,016 per year, as shown in Figure 18.
| FIGURE 18. SUMMARY BOP AND OIG COST-BENEFIT ANALYSIS |
|
| Source: BOP and OIG survey and analysis |
In our judgment, it is important that the BOP has an accurate understanding of the budgetary impacts of its Central Fill proposal before proceeding. Therefore, we recommend that it conduct a complete and accurate cost benefit analysis considering the monetary and non monetary impacts of the proposal before deciding if it should proceed with the implementation of this proposal.
Additional Concerns about the Central Fill Cost-Benefit Analysis
In addition to the concerns related to the specific dollar estimates, we identified several other concerns related to the BOP's Central Fill cost-benefit analysis. These include: (1) the representation of the six institutions used to estimate savings, as compared to the BOP as a whole; (2) whether pharmacists ensure that the lowest-cost prescription medications are purchased; (3) the static nature of the analysis which does not include any assumptions of growth in inmate population or changes in prescription medication costs; and (4) the increase in clinical work conducted by pharmacists.
In our judgment, the six institutions used by the BOP to estimate prescription medication costs may not represent the average institution. The BOP stated that it picked six institutions at random; however, no sampling methodology was used to ensure that the sample was representative of all institutions. Although we could not verify the validity of the sample, we found several factors that cause concern. The average amount spent on prescription medications by the six institutions was 23 percent higher than the average for all other BOP institutions. Further, 47 percent of the FY 2004 prescription medication costs for one of the six institutions were for Hepatitis C medications, compared to an average of 3 percent for all BOP institutions. These issues raise concerns about the BOP's ability to project analyses of these six institutions to all BOP institutions.
According to the Workgroup, Central Fill would allow the BOP to utilize VA staff to research manufacturer prices and purchase prescription medications at the lowest cost. The BOP asserts that using the Central Fill would result in cost savings because BOP pharmacists currently do not have the time to conduct price research. However, we found that it is possible for BOP pharmacists to research the lowest prescription medications prices. We reviewed prescription medication purchases at several BOP institutions and found significant price differences for identical medication during the same time period. For example, one institution purchased Nasalide® 25 mcg nasal spray on October 14, 2003, for $0.98 each, while another institution purchased the same item on October 21, 2003, for $0.21 each, for a difference of $0.77 per item. This demonstrates that BOP pharmacists can research the lowest prescription medication prices. Thus, in our judgment the BOP should implement a policy that would require staff to search for the lowest possible price within the FSS.
We also found that the BOP's cost-benefit analysis was based on the number of BOP institutions, number of prescriptions, inmate population, and prescription medication costs in FY 2004, without taking into account future changes. Based on the current implementation plan for Central Fill, the BOP does not anticipate full implementation until December 2009. As a result, many of the factors used to compile the analysis may change and should be incorporated to develop a complete and accurate cost estimate. For example, cost estimates do not include information regarding inmate population growth, changing demographics of the inmate population, increasing prescription medication costs, and BOP staffing levels, since each of these factors impact the estimated savings. We recommend that they should be included in the cost analysis if the BOP is to accurately project the proposal's future impact on the prescription medication costs.
In addition, as stated in the Introduction section of this report, the BOP Workgroup asserts the Central Fill proposal will increase pharmacists' ability to conduct clinical work. The BOP asserts that the proposal frees time, allowing pharmacists to hold clinics to better manage disease and medication through education and examinations. The free time occurs because pharmacists will no longer have to fill prescription bottles manually. We believe that the BOP may be overestimating the amount of time Central Fill will actually save pharmacists. Out of the 12 institutions visited, 10 would use the Central Fill for the majority of their prescriptions. Of these 10, 8 had at least 1 pharmacy technician, who usually filled prescription bottles manually. By eliminating the need to fill the prescriptions at the institution, the BOP is reducing the workload of the pharmacy technicians rather than the pharmacists. The pharmacists currently spend much of their time reviewing prescriptions for contraindications and effectiveness, which will still be completed by the BOP pharmacists under Central Fill, resulting in little reduction of their workload.
In sum, the BOP's cost-benefit analysis overstates the savings related to the Central Fill proposal and fails to consider other important issues. The BOP estimated that a fully implemented Central Fill would save $1.14 million per year, while our analysis shows that Central Fill could actually cost approximately $900,000 per year. It is important that the BOP fully consider the monetary and non-monetary impacts of the proposal before deciding if it should proceed with the implementation of this proposal.
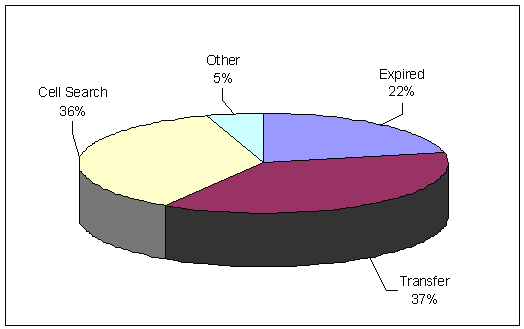
We found that the BOP needs to improve efforts to reduce prescription medication costs associated with waste. Based on the responses to our pharmacist's survey, we found that the BOP pharmacist estimated prescription medication costs associated with waste at $2.81 million in FY 2004, or 5.54 percent of the BOP's total prescription medication costs. Most often, the survey found that prescription medication waste is the result of inmate transfers and confiscations. As shown in Figure 19, waste from transfers and confiscations during searches of inmates' cells comprise approximately 74 percent of total waste, an estimated $2.07 million per year.
| FIGURE 19. | DISTRIBUTION OF PRESCRIPTION MEDICATION WASTE BY TYPE |
 | |
| Source: OIG Survey of BOP Pharmacists | |
The results of our pharmacist survey showed that the transfer of inmates is the largest cause of prescription medication waste, accounting for an estimated $1.05 million annually. Waste from inmate transfers results from the BOP's policy requiring that all transferred inmates receive a 7-day supply of prescription medications, regardless of whether or not the inmate already has a sufficient supply. This policy was established to ensure enough prescription medication is available to the inmate during the transfer and to give the new institution time to purchase the medication if it is not currently in the institution's inventory. There is currently no BOP requirement that prescription medications already in the inmate's possession be transferred with the inmate. As a result, when inmates are transferred, their prescription medications are often left in their cell or locker. In turn, these medications must be disposed of since the pharmacy cannot reuse them for another inmate once it has been in an inmate's possession.
In response to our survey, pharmacists offered several suggestions to reduce waste from transfers. Out of 81 pharmacists who responded to this question, 37 (46 percent) suggested that the BOP require medications to be transferred with the inmates. Other suggestions include: (1) limiting the amount of medication dispensed to inmates; (2) requiring correctional officers to return medication to the pharmacy prior to transfer; and (3) shortening the number of days of medication required for intra-system transfers from 7 to 3 days.
Our pharmacist survey responses indicated that confiscations during searches of inmates' cells were the second largest reason for prescription medication waste. Confiscations from waste totaled an estimated $1.02 million in FY 2004. Waste from confiscations was generally related to the BOP's policy prior to January 15, 2005, that prescriptions could only be valid for a total of 90 days (30 days with 2 refills). Therefore, the expiration date on the prescription label was for no more than 90 days, even though the medication may still be valid per the manufacturers' expiration date and still valid for use by the inmate. During searches of inmates' cells, if correctional officers found prescription medications that were past the expiration date per the label, the medications were confiscated and frequently thrown away.
Based on our survey, BOP pharmacists noted that if correctional officers were required to return confiscated prescription medications to the pharmacy, there was a possibility that the medication could be reissued to the same inmate. In addition, this would assist the pharmacists in tracking inmate prescription medication usage. The BOP revised its policy on January 15, 2005, and extended the length of time for a valid prescription from 90 to 180 days (a 30-day prescription with 5 refills), which may reduce waste resulting from confiscations.
In conclusion, prescription medication waste represents a significant cost to the BOP, representing about 5 percent of total prescription medication expenditures. The BOP has made some progress addressing these issues by extending the expiration date, which may help to reduce the waste associated with confiscations. However, we recommend the BOP implement policies and procedures ensuring the transfer of prescription medications with inmates, and that confiscated prescription medications are returned to the pharmacy for reissuance or disposal.
Over-the-Counter Medication Program Statement
In an effort to reduce the cost of prescription medications, the BOP issued the OTC Medication Program Statement, on November 17, 2004 . This statement outlines the requirements that each institution – other than the medical centers' inmates who are classified as in-patient status – must follow when using OTC medications for the treatment of inmates. The BOP requires that inmates who complain about cosmetic, general hygiene, or symptoms of minor ailments should be referred to the commissary, where they can purchase OTC medications with their own funds. If an inmate is considered indigent, that is, having less than a $6.00 average balance in their account for the last 30 days, then the institution must provide two OTC medications per week to the inmate, as needed. The following OTC medications are available to BOP inmates, as shown in Figure 20.
| FIGURE 20. THE BOP OTC MEDICATIONS |
|
| Source: | BOP Program Statement No. 6541.02, Over-the-Counter Medications, issued November 17, 2004 |
However, based on our review of 12 BOP institutions and our pharmacist survey, we found that the BOP has not fully implemented OTC policy throughout BOP institutions. Specifically, 35 percent of the respondents stated on our survey that the OTC policy had not been followed at their institution. Additionally, 43 percent of respondents stated that medical staff directed them to provide OTC medication to an inmate even though it was either not medically necessary or could be obtained from the commissary.
The savings generated from implementing the OTC policy relates to time, instead of dollar savings. OTC medication comprises only 2.7 percent, or $1.36 million, of the BOP FY 2004 medication costs. As a result, lowering the amount of OTC medication issued has a small impact on the total costs. However, the larger savings from this policy relates to pharmacists' time. We found pharmacists and their staff spend much of their day reviewing and filling prescriptions for all medications. By shifting the OTC medication from the pharmacy to the commissary, the BOP will reduce the number of prescriptions the pharmacy staff has to review and fill on a daily basis. This will allow pharmacists more time to complete other required tasks.
We recommend that the BOP:
- Conduct a complete and accurate cost-benefit analysis of the Central Fill proposal before deciding whether to proceed with implementation.
- Pursue efforts to request that Congress amend Pub. L. No. 102-585 (1993) to provide the BOP with eligibility for Big 4 pricing.
- Ensure BOP pharmacy staff search for the lowest possible prescription medication prices within the FSS.
- Implement a system that would ensure that prescription medications are transferred with the inmates, by taking into account security issues.
- Ensure that prescription medication confiscated from an inmate is returned to the pharmacy for reissuance to the same inmate or is disposed of properly.
- Ensure that all BOP institutions comply with the OTC policy.
II. CONTROLS AND SAFEGUARDS OVER PRESCRIPTION MEDICATIONS
- The BOP is not adequately accounting for and safeguarding prescription medications. As a result, the BOP could not account for 402 controlled substances at the institutions included in our audit. In addition, we noted numerous errors related to controlled substances inventory and administration records. Furthermore, quarterly inventories submitted to BOP headquarters did not always include all controlled substances. We also found the BOP has not implemented adequate internal controls related to the purchasing, ordering, receiving, payment, and dispensing of prescription medications.
BOP policy requires that necessary medical, dental, and mental health services be provided to inmates by professional staff.31 Sick or injured inmates may be seen during routine appointments or through sick calls at the institution. If an inmate needs a prescription medication, the health service practitioner prepares a written order, which can sometimes include controlled substances.
Controlled substances are prescription medications that fall under the jurisdiction of federal and state laws regulating their manufacture, sale, distribution, use, and disposal. The federal government separates controlled substances into five schedules as defined by the Controlled Substances Act § 202 (21 USC 812), as follows:
- Schedule I – controlled substances that have no accepted medical use in the United States.
- Schedule II – controlled substances that have accepted medical uses but a high potential for abuse, with severe psychological or physical dependency.
- Schedule III through V – controlled substances that have accepted medical uses for which the potential for abuse is in decreasing levels of dependency.
The Drug Enforcement Agency (DEA) is the primary agency responsible for enforcing the Controlled Substances Act. In order to prescribe and administer controlled substances, institutions and physicians must register and obtain a DEA Controlled Substance Registration Certificate. The DEA requires registrants to comply with regulatory requirements related to security, records accountability, and other specific standards for controlled substances. Specifically, the DEA requires that registrants conduct biennial inventories of all controlled substances and maintain records of all purchases, disposals, and administration of controlled substances for at least 2 years. Registrants must also have a written prescription for controlled substances that include the physician's signature and applicable DEA registration number. Each BOP institution is registered with the DEA to prescribe, dispense, and administer controlled substances.32
The BOP places further requirements on the use and accounting for controlled substances.33 BOP policy requires that the mainstock of controlled substances be stored in a safe or vault and that only the Chief Pharmacist or designee should have access.34 Each institution is required to maintain a perpetual inventory for all controlled substances stored in the mainstock. The inventory for controlled substances is used to record all purchases, transfers, and disposals of controlled substances in and out of the mainstock.
BOP policy also requires that any substocks of controlled substances be stored in a stationary, approved steel cabinet with overlapping steel doors that are separately locked or in a safe with a key padlock. Several institutions use an automated system, such as a Pyxis or OmniCell system, to store controlled substances substock. These systems allow for improved electronic tracking and recordkeeping through inventory records. The only staff members who generally have access to the substock are pharmacists, pharmacy technicians, and mid-level practitioners. According to BOP policy, institutions should maintain a perpetual inventory of controlled substances in any substock. In addition, institutions are required to count controlled substances at each shift change.
Each time a controlled substance is administered to an inmate from the substock, the BOP requires it to be recorded on the Proof of Use sheet as well as the inmates' Medication Administration Record (MAR). The Proof of Use sheet is the substock inventory record and includes the inmate's name and number, date issued, strength, quantity, signature of the person administering the medication, and balance on hand. The MAR should include the prescription medication name, strength, quantity, date, time, and person who administered the prescription medication. The information on the Proof of Use sheet and the MAR should agree.
At the 12 institutions included in our audit, we identified 22 different types of controlled substances that were being used to treat inmates. (See Appendix I for the medical uses of each type of controlled substance.) Tylenol® with Codeine and Phenobarbital were the most commonly used controlled substances throughout the institutions. The types of controlled substances identified in our audit, the number of institutions using each type, and the DEA Schedule classification, are shown in Figure 21.
| FIGURE 21. | CONTROLLED SUBSTANCES IDENTIFIED AT THE 12 BOP INSTITUTIONS AUDITED |
|
| Source: OIG analysis of BOP institutions' inventory records |
At each institution audited, we reviewed all relevant documents for controlled substances to account for every dose. We conducted an accountability audit consisting of a physical count of controlled substances during our review, and a review of all mainstock and substock records for a 1-year period. This included a review of documents related to purchases, disposals, administrations, and transfers. At the Springfield Medical Center we judgmentally selected a sample of nine controlled substances, and reviewed at least a 7-month period because of the large volume of use at the institution.35
Based on the results of our accountability audit, we found that BOP institutions did not always adequately account for and safeguard controlled substances. Specifically, we identified 402 unaccounted for doses of controlled substances out of a total of 42,125 that should have been on hand at the time of our inventory as shown in Figure 22.
| FIGURE 22. | UNACCOUNTED FOR CONTROLLED SUBSTANCES BY BOP INSTITUTION |
|
| Source: OIG analysis of BOP Institutions |
A summary of our findings related to the missing controlled substances by institution is included in Appendix I.
Controlled Substance Recordkeeping Errors
Additionally, we found numerous errors in the controlled substances inventory records, which based on the inventory records alone, appeared to result in unaccounted controlled substances. We were able to resolve all of these discrepancies by reviewing additional documents. Although, these errors did not result in missing controlled substances, errors in the inventory records for controlled substances weaken the ability of the BOP to accurately account for and safeguard the controlled substances.
At the 12 institutions included in our audit, we reviewed the controlled substances records for the mainstock perpetual inventory and substock Proof of Use sheets to determine if the required information was complete and accurate.37 As a result of our review, we identified two different types of errors relating to the records used to account for controlled substances: (1) errors that resulted in discrepancies in the controlled substances balance that could be resolved using inmates' MARs, and (2) missing or incomplete information in the mainstock and substock records, such as date, time, inmate number or name, and signature of person administering the medication. These errors were generally related to substock Proof of Use sheets, which are maintained by health services staff administering the controlled substances, and not the pharmacist. We noted many errors had been identified previously as deficiencies during the institutions' Operation and Program Reviews.
Specifically, we identified approximately 400 recordkeeping errors on the controlled substances inventories that appeared to result in unaccounted-for controlled substances. However, we were able to account for the controlled substances using additional documentation. The following types of errors comprise the majority of these issues.
- Transfer location not identified – we noted 133 instances where the transfer location was not identified in the mainstock or substock inventory;
- Incorrect number in the usage column – we noted 52 instances where the incorrect amount was entered into the usage column on the Proof of Use sheet;
- “Floor charge” entries – we noted 46 instances where a prescription medication was removed from the OmniCell and shown as a “floor charge” rather than as an administration to a specific inmate, which would include the inmate's name and number; and
- Missing entry or amount in the usage column – we noted 77 instances where the amount administered was not entered in the usage column on the Proof of Use sheet.
We also noted the following errors on the inventory records for controlled substances:
- incorrect beginning inventory balance,
- incorrect dose subtracted from the balance column,
- doses administered that were not recorded,
- duplicate entries,
- incorrect strength shown for medication,
- inventory overstated,
- purchase not recorded in mainstock,
- disposal not recorded in mainstock,
- transfer not recorded in mainstock,
- incorrect transfer amount recorded in mainstock, and
- substock records show administered to pill line rather than to inmate.
In addition to these recordkeeping errors, we noted our physical count of controlled substances sometimes exceeded the balance on the inventory records. For example, at one institution we identified 32 extra Phenobarbital tablets from mainstock records that may have been caused by an unrecorded transfer of controlled substances with an inmate who transferred into the institution. There were also instances where the Proof of Use sheet had incorrect information, resulting in a physical count that exceeded the balance per the inventory record.
We also identified approximately 800 instances for which required information was not entered in the mainstock and substock inventory records, including the following:
- missing inmate name and, or number;
- missing inmate prescription medication number;
- missing date and time the prescription medication was administered; and
- missing signature of the person administering the prescription medication.
As stated previously, these errors weaken the ability of the BOP to adequately account for and safeguard controlled substances, which can result in missing or stolen medication. For example, as a result of inadequate recordkeeping, there was a theft of controlled substances from the Springfield Medical Center. According to an OIG Investigation, between June and December 2003, a Licensed Practical Nurse stole approximately 1,300 Percocet®, which the DEA labels a Schedule II controlled substance. The nurse was convicted in the United States District Court, Springfield, Missouri, for violation of Title 21, USC 844(a), Possession of Controlled Substances and sentenced to 3-years probation.
The investigation found that the nurse concealed the theft by altering and destroying various records for the controlled substances, because of poor recordkeeping at the facility. In fact, she concealed the theft for approximately 6 months. The nurse identified several factors that also contributed to her ability to steal the controlled substances. She noted that there were no supervisors or other independent persons that verified the controlled substances log sheets. In addition, the Proof of Use sheets were not routinely returned to the pharmacy in a timely manner, and when they were returned, the nurse did not believe that pharmacy staff reviewed the sheets to check for errors.
Controlled Substance Inventory
The DEA requires that each registrant conduct a biennial inventory of all controlled substances. In addition, the BOP requires that the mainstock is inventoried quarterly with results submitted to BOP headquarters, and that the substock is inventoried at each shift change, unless the institution is using an automated system.
According to federal regulations, “Each inventory shall contain a complete and accurate record of all controlled substances on hand on the date the inventory is taken . . . . Controlled Substances shall be deemed to be 'on hand' if they are in the possession of or under the control of the registrant, including substances returned by a customer."38 We found quarterly inventories submitted to BOP headquarters did not always include all controlled substances. Specifically, we identified controlled substances at three institutions that should have been listed on the mainstock in the quarterly inventory:
- Atwater USP – 70 Lomotil® tablets located within a lock box in the pharmacy were not included in any inventory;
- La Tuna FCI – 53 Klonopin® tablets that were transferred in with an inmate but not included in the institution's quarterly controlled substances inventory; and
- Florence FCI – 7.2 milliliters of Andro® that were transferred in with an inmate but not included in the institution's quarterly controlled substances inventory.
We also found that 10 out of 12 institutions audited did not include controlled substances in substock quarterly inventories. Pursuant to BOP policy, the institutions are only required to include mainstock in the quarterly controlled substances inventories.39 However, federal regulations require all controlled substances to be included in the inventories required by the DEA. Given the numerous recordkeeping errors related to controlled substances noted in this report, in our judgment, we believe it is important for the BOP to conduct a complete accounting of all controlled substances on a quarterly basis.
Administration of Controlled Substances
According to BOP policy, health service staff members, including mid level practitioners, and medical and pharmacy technicians who have completed a Pharmacy Services Training Program can administer doses of controlled substance medications to inmates. The staff member administering the medication is required to record it on the Proof of Use sheet and the MAR, immediately following administration of the controlled substance. Information recorded on the Proof of Use sheet must include the inmate's name, inmate number, date, time, dosage administered, and signature of the person who administered the controlled substance. The information recorded on the MAR, which is located in the inmate's medical file, should include the name, quantity, and strength of the controlled substance issued, as well as the date, time, and name of the person who administered the controlled substance.
At each of the 12 institutions included in our audit, we generally selected a sample of 20 controlled substances administered to inmates from the Proof of Use sheets except for Oklahoma FTC during the period September 2003 through April 2005. Our sample included a total of 245 administrations of controlled substances to inmates based on the information recorded on the Proof of Use sheets. We then compared the information recorded on the Proof of Use sheet to the inmate's MAR to verify that the inmate received the medication, and we determined if the institutions were in compliance with the BOP's requirements.
As shown in Figure 23, we found that the institutions did not always comply with the BOP's requirements for administering controlled substances to inmates.
| FIGURE 23. ADMINISTRATION OF CONTROLLED SUBSTANCES REVIEW |
|
| Source: OIG analysis of the BOP's review of the administration of controlled substances |
Specifically, we found:
- for 26 controlled substance administrations, we were unable to locate the MAR in the inmate's medical file that covered the period during which the controlled substances were recorded as being administered; as a result, there are no assurances that the inmate received the medication;
- for 18 controlled substance administrations, the date of the administration per the inmate's MAR did not match the date recorded on the Proof of Use sheet; and
- for 17 controlled substance administrations, incorrect information was recorded on the Proof of Use sheet, including the inmate's name, or the information recorded on the MAR including prescription information and the dose administered did not match the Proof of Use sheet, or the inmate was given a medication without obtaining the required non formulary waiver.
It is important that the required administration information of controlled substances is complete and accurate on the Proof of Use sheet and the MAR. Without accurate documentation the institution has no assurance that the medication was administered to the correct inmate or in the correct dose. This weakens the institution's ability to account for or to detect theft of controlled substances.
Prescription Medications Purchasing
The BOP purchases its prescription medications through a “prime vendor” contract that is administered by the VA. During our audit, we identified inadequate internal controls related to purchasing, ordering, receiving, and paying for prescription medications. At each institution included in our audit, we found that there was no evidence of any segregation of duties related to purchasing of prescription medications. At 4 of the 12 institutions, either the Health Services Administrator (HSA) or the contracting officer signed a blanket purchase agreement for the entire year and delegated all responsibilities for ordering, receiving, and approving invoices for payment to the pharmacist. In addition, 5 of the 12 institutions audited, there was no documentation that the pharmacist verified the prescription medications received to the invoice. Furthermore, in 5 of the 12 institutions the person who ordered and received prescription medications was also the individual who signed off on the invoice before it was submitted to the business office for payment.
The lack of internal controls over the purchasing of prescription medications at BOP institutions allowed a Chief Pharmacist at the El Reno FCI to illegally purchase four different brands of non-formulary prescription medications. An OIG investigation found that he stole a total of 30,600 doses between July 2002 and February 2004, for his personal consumption. This individual's purchases included Fioricet®, Soma®, Ultram®, and Mobic®, and cost the BOP approximately $1,567 with a retail value of approximately $28,700. In lieu of prosecution, the Western District of Oklahoma offered the pharmacist a 1-year Pretrial Diversion Program and if all conditions are met, he will not be prosecuted. Adequate segregation of duties for purchasing and receiving of controlled substances would likely have prevented this theft.
We recommend the BOP:
- Ensure all documentation related to controlled substances is accurate and complete.
- Require all controlled substances, including those in the substock, crash cart, and other locations, are included in the quarterly inventories.
- Develop and implement policies and procedures requiring the Health Service Administrator or an authorized designee to approve each prescription medication purchase order and verify the items received to each vendor invoice.
- Develop and implement policies and procedures requiring adequate segregation of duties for ordering and receiving prescription medications.
- We found that the BOP pharmacies were not always in compliance with applicable BOP policies and procedures. At the 12 BOP institutions included in our audit, we found that 384 (35 percent) of the prescriptions reviewed were not in compliance with BOP policy. Specifically we found prescriptions for which: (1) the pharmacist's or physician's signature was not documented; (2) the separate written prescription form for controlled substances was not maintained; (3) required information was missing in inmates' medical files; (4) controlled substance prescription forms were missing a DEA registration number or required signature; and (5) the prescription time period exceeded the BOP policy for controlled substances. We also found 31 prescriptions (84 percent) for non-formulary medications and 14 purchases (47 percent) of non-formulary medications for which the required waivers were not obtained.
In assessing compliance with BOP policies and procedures, we judgmentally selected a sample of about 100 prescriptions written from October 2003 through July 2005 at each institution audited, with the exception of the Oklahoma FTC.40 Our sample generally consisted of 50 controlled and 50 noncontrolled substances prescriptions. If an institution did not have 50 prescriptions for controlled substances during the period, we increased our sample of noncontrolled substances for a total combined sample of about 100 prescriptions. Additionally, if the prescription selected from our sample was for an inmate that had been transferred or released, we verified the inmate's location by using the BOP's SENTRY system and replaced the sample with a new prescription since medical files are transferred with the inmates.
At the 12 institutions included in our audit, we selected a total of 1,107 prescriptions, including 488 prescriptions for controlled substances. For each prescription in our sample, we reviewed the inmate's medical file to ensure that the appropriate information such as prescription name, dosage, and instructions were recorded, and that the pharmacist's review was documented. In addition, we reviewed prescriptions for controlled substances to determine if separate prescription forms were completed, including all required information, and that the prescription was written for the allowable time period. We also reviewed prescriptions for non-formulary medications to determine if the required waivers had been obtained.
As shown in Figure 24, we found that 384 (35 percent) of the 1,107 prescriptions reviewed were not in compliance with applicable policies and procedures.
| FIGURE 24. PRESCRIPTION REVIEW |
|
| Source: OIG analysis of the BOP's prescription review |
We found 206 prescriptions for which the pharmacist's review for contraindications or physician's signature was not documented in the inmate's medical file. The majority of these prescriptions (93) were at the Atlanta USP. Officials there stated that pharmacists review prescriptions for contraindications but do not annotate their review in the inmate's medical chart because of a staffing shortage. At several other institutions, the pharmacist's review for contraindication was not documented because the prescription was faxed to the pharmacy. For example, at the Springfield Medical Center we found about 40 prescriptions that were faxed to the pharmacy from a nursing station and the faxed copy with the pharmacist's signature could not be retrieved. We also noted that as a result of faxing the prescriptions for contraindications, the pharmacist does not have the inmate's medical chart, as required, to conduct a review of the prescription for contraindications.
Generally, at each of the 12 institutions, we found that the written prescriptions were included in the inmate's medical files and that the prescriptions contained the required information; however, we noted that 19 prescriptions were not in the inmate's medical file or lacked required information such as prescription name, dosage, and instructions.
As stated previously, our sample included 488 prescriptions for controlled substances. According to federal regulations, all prescriptions for controlled substances are required to include the physician's name, address, signature, and DEA registration number.42 In addition, BOP policy requires that prescriptions for Schedule II controlled substances should be written for no more than 72 hours and that prescriptions for Schedule III through V controlled substances should not be written for more than 30 days, with up to 2 refills, not to exceed a total of 90 days.43 Based on our review of 488 controlled substance prescriptions, we found:
- 54 controlled substance prescriptions for which the prescription forms were missing a DEA registration number or required signature;
- 24 controlled substances prescriptions for which the required separate written prescription forms were not maintained by the institution; and
- 20 prescriptions that were written for periods exceeding 72 hours for Schedule II controlled substances or 90 days for Schedule III through V controlled substances.44
On February 10, 2005, the Director of BOP Pharmacy Services distributed an e-mail to BOP pharmacists stating that separate prescription forms are no longer required for non-receiving and discharging controlled substance medication orders. Instead, prescriptions for controlled substances can be filled directly from the inmate's medical file since all BOP pharmacies are licensed with the DEA as a hospital or clinic, which was verified with the DEA. However, the written prescription in the inmate's medical file must still contain the information required by federal regulations. In addition, BOP institutions are required to keep logs for prescription of controlled substances.
The BOP National Formulary (formulary) is a list of all prescription medications recommended as essential for inmate care and is used to help provide clinically appropriate, safe, and cost-effective prescription medications. The BOP Pharmacy and Therapeutics Committee, comprised of pharmacists and physicians from the institutions and the Central Office, develops and maintains the formulary. Physicians and pharmacists are generally required to limit prescriptions to those medications listed in the formulary. If a non-formulary prescription medication is deemed necessary for the treatment of an inmate, the prescriber is required to obtain a non formulary Drug Authorization (waiver) requesting approval for the use of non-formulary medication to treat a specific inmate's needs.45 The non formulary waiver must be approved by the BOP Medical Director.
For each of the 1,107 prescriptions included in our sample, we determined whether or not the medication was included in the BOP formulary. For any non-formulary medications, we determined whether the required non-formulary waiver had been obtained. Our sample of 1,107 prescriptions included 37 prescriptions that were for non-formulary medications. Of the 37 non-formulary prescriptions identified from our sample, we found that for 31 prescriptions (84 percent) the required non formulary waiver had not been obtained.46
In addition to reviewing the prescription sample to identify any non formulary prescription medications for which the required waivers were not obtained, we also reviewed a sample of medications purchased. At each institution included in our audit, we obtained invoices for prescription medications purchased from October 2003 through July 2005 and selected a sample of at least 100 individual medications. Our total sample consisted of 1,198 prescription medications purchased during the period of October 2003 through July 2005. We reviewed each prescription medication in our purchase sample to identify any non-formulary medications that were purchased and determined whether the required waiver had been obtained. Generally, the institutions included in our sample were in compliance with the BOP's requirements for non formulary prescription medications; however, we found a total of 30 purchases for non-formulary medications, of which 14 purchases (47 percent) did not obtain the required non-formulary waiver.
Although we found that the institutions included in our audit were generally in compliance with the BOP's non-formulary medication requirements, we did identify an issue related to the non-formulary waivers that should be addressed. The BOP's current requirements allow non formulary waivers to be issued for an indefinite period, which does not take into consideration changes in the inmate's medical condition or the availability of newer prescription medications. Therefore, in our judgment, the BOP should require that non-formulary waivers be renewed annually to ensure that the inmate's prescription is still medically necessary. In addition, since the BOP formulary is updated annually, this practice would allow the BOP to determine if another medication in the updated formulary may fulfill the need of the non-formulary prescription.
We recommend that the BOP:
- Implement a policy that requires pharmacist reviews for contraindications are documented in the inmates' files.
- Ensure that written prescriptions include all required information.
- Ensure that non-formulary waivers are obtained and required to be renewed annually.
- The six institutions were Forrest City FCI, Arkansas; Atlanta USP, Georgia; Petersburg FCI (Medium), Virginia; Oxford FCI, Wisconsin; Lompoc USP, California; and Fort Dix West FCI, New Jersey, from March 1, 2003, to February 28, 2004.
- To arrive at the 74.5 percent calculation we used the BOP's FY 2004 expenditures of $50.73 million and reduced this amount by: (1) 50 percent of the medical centers' and FDCs' costs ($11.00 million); (2) the entire FTC's costs ($0.51 million); and (3) 5 percent of the remaining BOP's costs ($1.41 million), which equal $37.81 million, or 74.5 percent ($37.81 million/ $50.73 million) of the total FY 2004 BOP prescription medication costs.
- Crash carts contain urgent care prescription medications, including controlled substances that are used for emergency purposes only.
- The calculation uses $47.72 million in cost because the Central Fill proposal affects all inventories of prescription medications within the BOP, not only the portion of prescription medication costs that will use the Central Fill ($35.55 million).
- The BOP assumes that institutions will use more labels than the actual number of prescriptions due to mistakes and various other reasons.
- To arrive at the 83.4 percent calculation we used the BOP's FY 2004 and FY 2003 average prescription of 3.33 million and reduced this amount by: (1) 50 percent of the medical centers' and FDCs' average prescriptions (0.43 million); and (2) 5 percent of the remaining BOP's prescriptions (0.12 million), which equal 2.78 million, or 83.4 percent (2.78 million/3.33 million). Note that the total BOP prescription numbers provided by the BOP did not include the prescriptions issued at the Oklahoma FTC.
- BOP Program Statement No. 6000.05, Health Service Manual, updated February 11, 2000.
- “Prescribing” is defined as writing or giving medical directions, or indicating remedies. “Dispensing” is defined as providing multiple doses in a properly labeled container for use over a period of time. “Administration” is defined as providing one dose of medication to be applied or consumed immediately.
- The Chief Pharmacist is responsible for procurement, storage, distribution, product selection, and security of all controlled substances.
- BOP Program Statement No. 6000.05, Health Service Manual, updated February 11, 2000.
- The Springfield Medical Center had 12 types of controlled substances consisting of 36 different strengths and forms (liquid vs. tablet).
- “Others” category includes, liquid doses in milliliters, injectables, and patches.
- We sampled 9 of the 36 controlled substances at the Springfield Medical Center.
- Code of Federal Regulations, 21 C.F.R. (2004) § 1304.11.
- These institutions are, Florence FCI, Florence USP, Alderson FPC, Atlanta USP, Atwater USP, Danbury FCI, Forrest City FCI (Low), Oklahoma FTC, Oxford FCI, and Springfield Medical Center.
- Over 80,000 inmates transfer through the Oklahoma FTC annually. Generally, the inmates are at the facility for an average of 14 to 30 days, and some inmates are at the facility for less than 24 hours. Therefore, we only reviewed a sample of 18 prescriptions at FTC Oklahoma because the medical files are transferred with inmates.
- The total number of findings may include multiple errors for the same prescription.
- Code of Federal Regulations, 21 C.F.R. § 1306.05 (2004).
- BOP Program Statement No. 6000.05, Health Services Manual, updated February 11, 2000.
- Schedule II controlled substances can be written for 30 days if the medication is used in cases of chronic or terminal illness resulting in unremitting pain not likely to abate in the short term, or if it is used to treat narcolepsy.
- BOP Program Statement No. 6501.06, Pharmacy Technical Reference Manual, updated February 28, 2001.
- This analysis includes prescriptions for each separate non-formulary prescription. For example, we found 23 instances where the same non-formulary medication was prescribed to the same inmate without the required waiver and this was counted as 23 separate non-formulary prescriptions without the required waiver.