The Federal Bureau of Prisons (BOP) is faced with a significant challenge in providing adequate and cost-effective medical care to inmates because of the rising federal inmate population and the increasing cost of prescription medications. The BOP's total health care costs for treating inmates increased from $412.65 million in FY 2000 to $623.52 million in FY 2004, an average annual increase of about 11 percent. During that same period, the BOP's costs for prescription medications and related supplies increased an average of 23 percent annually, from $22.51 million in FY 2000 to $50.73 million in FY 2004. Additionally, the cost of prescription medications and related supplies has continued to account for a growing share of the BOP's total health care costs, rising from 5.5 percent in FY 2000 to 8.1 percent in FY 2004.
The Department of Justice (DOJ) Office of the Inspector General (OIG) conducted this audit to:
- evaluate the BOP's efforts to reduce increasing costs of its prescription medications;
- assess whether the BOP ensures adequate controls and safeguards over prescription medications; and
- assess whether the BOP pharmacies are in compliance with applicable laws, regulations, policies, and procedures.
During the audit, we conducted work at the BOP headquarters and 12 BOP institutions, consisting of 4 Federal Correctional Institutions (FCI), 3 United State Penitentiaries (USP), 1 Federal Prison Camp (FPC), 1 Administrative Maximum Security (ADX), 1 Federal Transfer Center (FTC), and 1 medical center as shown in Figure 1.
| FIGURE 1. BOP INSTITUTIONS AUDITED |
|
Background
Health care costs consist of many different components. In 2002 the three largest components were hospital care (31 percent), physician and clinical services (22 percent), and prescription medications (11 percent). Of these components, prescription medication costs have grown at the fastest rate, increasing by 167 percent from 1995 to 2002. Two significant factors related to the rise in prescription medication costs are increased price and increased usage. From 1995 to 2002, the Consumer Price Index for prescription medications and medical supplies increased by 35 percent, while the Consumer Price Index for the United States, on average, increased by only 18 percent. Additionally, the number of individuals reporting that they had taken at least one prescription medication in the month prior to the survey increased from 39 percent in 1988 through 1994, to 44 percent in 1999 through 2000.1
As of July 2005, the BOP was responsible for the custody and care of approximately 182,000 federal offenders. The BOP consisted of 106 institutions, 6 regional offices, a central office, 2 staff training centers, and 28 community corrections management offices.
The BOP's daily prescription medication cost was $0.92 per inmate in FY 2004, an increase of 5 percent from FY 2003, and 79 percent from FY 2000. The BOP attributes the increase in its prescription medication costs to various reasons, including the: (1) increase in inmate population, and (2) increasing prices of prescription medications as shown in Figure 2.
| FIGURE 2. | CHANGES IN PRESCRIPTION MEDICATION COSTS AND INMATE POPULATION2 |
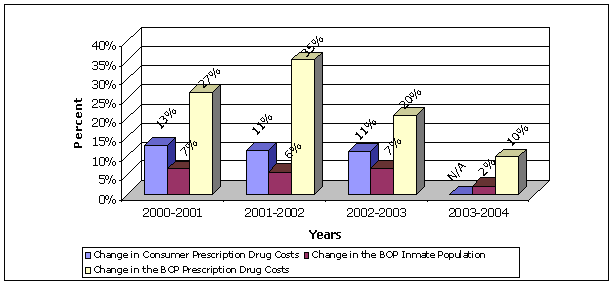 | |
| Source: Inmate population and prescription medication costs provided by the BOP; consumer costs obtained from the U.S. Statistical Abstract 2004-2005. | |
In an effort to reduce prescription medication costs, the BOP is planning to or has implemented the following proposals:
- Levels of Care – through Levels of Care, BOP institutions will be classified based on the level of medical care required by the inmates. The classification will include four levels based on the severity of inmates' medical needs, with Level 1 consisting of healthy inmates and Level 4 consisting of inmates at one of the BOP's six medical centers. In turn, the BOP plans to reorganize the staffing of its pharmacies to reflect the medical classification of its institutions.
- Central Fill – through Central Fill, pharmacists at BOP institutions will review prescriptions to ensure an inmate is not allergic to a medication, and that a medication does not negatively interact with an inmate's current prescriptions or medical condition. The pharmacist will then transmit the prescriptions electronically to the Department of Veterans Affairs (VA) Centralized Mail Outpatient Pharmacy Center in Dallas, Texas. The VA will fill the prescriptions and mail them overnight to the institution.
- Central Processing – through Central Processing, institutions that do not have a pharmacist on site will be able to electronically transmit the inmate's prescriptions to a central location, where BOP pharmacists will review them for contraindications and then transmit them electronically to Central Fill.3
- Electronic Medical Records System – through an electronic medical records system, BOP pharmacists will be able to access the inmate's medical information from any location, thus allowing them to conduct a complete review of inmate prescriptions to check for any contraindications. In addition, the system will allow for prescriber order entry, so that physicians can electronically enter prescriptions into the system.
- Over-the-Counter (OTC) Policy – the OTC policy outlines the requirements that each BOP institution, except medical centers, must follow when using OTC medications for the treatment of inmates. The BOP's OTC policy requires that inmates who complain about cosmetic, general hygiene issues, or symptoms of minor ailments should be referred to the commissary where they can purchase OTC medications with their own funds.
Summary of OIG Findings
Our audit concluded that, the BOP has not adequately assessed the budgetary impact of its initiatives to reduce increasing costs for prescription medications. As a result, future initiatives may result in increased, rather than decreased costs. We also found that the BOP's cost-benefit analysis for its Central Fill proposal contained errors and incorrect assumptions that may result in increased prescription medication costs rather than savings. We also found that the BOP needs to improve efforts to reduce prescription medication costs associated with waste and ensure that cost savings initiatives such as the OTC policy are fully implemented.
Cost Savings Initiatives
The BOP completed a cost-benefit analysis of its Central Fill proposal in March 2004, to estimate the impact on the BOP's prescription medication costs. Based on its cost-benefit analysis, the BOP estimated that Central Fill will result in a savings of $1.14 million per year. However, based on our analysis, we concluded Central Fill may cost the BOP as much as $895,016 more per year, as shown in Figure 3.
| FIGURE 3. SUMMARY BOP AND OIG COST-BENEFIT ANALYSIS |
|
| Source: BOP and OIG survey and analysis |
As shown in Figure 3, the BOP estimated that Central Fill would result in gross annual savings of $7.74 million, annual costs of $6.6 million, and annual net savings of $1.14 million. Based on our analysis, the BOP may have overstated annual gross savings by $4.97 million and annual gross costs by $2.93 million, resulting in overstated annual net savings of $2.03 million. Specifically, we found that:
- The data used by the BOP to calculate the gross purchase savings of $4.94 million included two errors that resulted in overstated savings of $2.3 million.
- The BOP's analysis used to calculate gross purchase savings also incorrectly assumed that all institutions will use Central Fill for 100 percent of prescription medications, resulting in additional overstated savings of $0.67 million.
- The BOP estimated savings of $2.39 million from the reduction of waste of prescription medications. However, based on our survey of BOP pharmacists, we estimated savings related to waste of only $0.58 million, resulting in overstated savings of $1.81 million.
- The BOP estimated that Central Fill will reduce the costs related to vials and labels by $0.41 million per year. However, this figure was based on the incorrect assumption that all institutions will use Central Fill for 100 percent of prescription medications, resulting in overstated savings of $0.18 million.
- The BOP estimated that VA fees for filling prescriptions would cost $5.6 million annually. However, this estimate was also based on 100 percent usage of Central Fill for prescription medications, resulting in overstated costs of $2.49 million.
- The BOP estimated costs of $1 million annually for shipping. However, this estimate was based on 100 percent usage of Central Fill for prescription medications, resulting in overstated costs of $0.45 million.
In summary, the BOP's cost-benefit analysis for its Central Fill proposal includes several errors and incorrect assumptions. As a result, the BOP's estimate that Central Fill will result in net annual savings of $1.14 million is incorrect. Based on our analysis, we found that Central Fill may actually increase prescription medication costs by approximately $900,000 per year. Therefore, it is essential that the BOP has an accurate understanding of the budgetary impacts of the Central Fill proposal before proceeding with implementation.
We also concluded that the BOP needs to improve efforts to reduce prescription medication costs associated with waste. Based on the responses to our survey of BOP pharmacists, we found that prescription medication costs associated with waste were estimated at $2.81 million in FY 2004, or 5.54 percent of the BOP's total prescription medication costs.
Based on the results of our pharmacist survey, the transfer of inmates is the largest reason for prescription medication waste, accounting for an estimated $1.05 million in FY 2004. Waste from inmate transfers results from the fact that all inmates who are transferred receive a 7-day supply of their prescription medications regardless of whether or not the inmate already has a sufficient supply. In addition, there is currently no BOP requirement that prescription medications already in the inmate's possession are transferred with the inmate. As a result, when inmates are transferred their prescription medications are often left in the inmate's cell or locker and must be disposed of because the pharmacy cannot reuse medication once it has been in an inmate's possession.
Confiscations during searches of inmates' cells were the second largest reason for prescription medication waste based on our pharmacist survey, accounting for an estimated $1.02 million in FY 2004. Waste from confiscations was generally related to the BOP's policy prior to January 15, 2005, that prescriptions could only be valid for a total of 90 days (30 days with 2 refills). Therefore, expiration dates on prescription labels indicated 90 days or less, even though the medication may still be valid according to the manufacturer's expiration date. During searches of inmates' cells, if correctional officers find a prescription medication that is past the expiration date on the label, the medication is confiscated and frequently thrown away. In our survey, BOP pharmacists noted that if correctional officers were instructed to return confiscated prescription medications to the pharmacy, some of the medications could be reissued to the same inmates. In addition, this would assist the pharmacists in tracking inmate prescription medication usage.
In an effort to reduce prescription medication waste and save pharmacist time, the BOP issued the OTC Medication Program Statement on November 17, 2004. The BOP's OTC policy requires that inmates who complain about cosmetic, general hygiene issues, or symptoms of minor ailments should be referred to the commissary where they can purchase OTC medications with their own funds. However, based on our review of 12 BOP institutions and our pharmacist survey, we found that the OTC policy has not been fully implemented or consistently applied throughout the BOP institutions. Specifically, our survey found that, as of April 2005, 35 percent of the respondents stated that the OTC policy had not been implemented at their institution. Additionally, 43 percent of the survey respondents stated that they had been told by medical staff to provide OTC medication to an inmate even though it was either not medically necessary or could be obtained from the commissary by the inmate.
Controls and Safeguard over Prescription Medications
Our audit found that, the BOP is not adequately accounting for and safeguarding prescription medications. As a result, the BOP could not account for 1 percent of the controlled substances that should have been on hand at the time of our inventory at the institutions included in our audit. However, unaccounted for controlled substances within institutions identify issues related to internal controls that undermine the accounting and safeguarding of prescription medications. In addition, we noted numerous errors related to controlled substances inventory and administration records. For instance, quarterly inventories submitted to BOP headquarters did not always include all controlled substances. We also found the BOP has not implemented adequate internal controls related to the purchasing, ordering, receiving, payment, and dispensing of prescription medications.
At each of the institutions included in our review, we conducted an accountability audit of controlled substances. The accountability audit consisted of a physical count of controlled substances at the time of our visit and a review of all mainstock and substock records4 for the 1-year period prior to our audit, including an analysis of documentation related to purchases, disposals, administrations, and transfers.5 As a result of our audit, we identified 402 unaccounted for doses of controlled substances out of a total of 42,125 that should have been on hand at the time of our inventory at the 12 institutions audited.
Additionally, we found numerous errors in the controlled substances inventory records, which based on the inventory records alone appeared to result in unaccounted-for controlled substances. However, we were able to resolve these discrepancies by reviewing additional documentation. Specifically, we identified approximately 400 inventory recordkeeping errors related to: (1) transfer location was not identified in the mainstock or substock inventory; (2) no amount administered or an incorrect amount administered was entered into the usage column; and (3) the administration was entered as a “floor charge” rather than to a specific inmate, identified by inmate name and number. We also identified approximately 800 recordkeeping errors related to missing information, including inmate names, inmate numbers, prescription numbers, dates, and times that medications were administered.
In addition to conducting an accountability audit of controlled substances, we selected a total of 245 controlled substances administered to inmates from the Proof of Use sheets and compared the information to the inmate's Medication Administration Record (MAR) to verify that the inmate received the medication.6 Based on our review, we found that 25 percent of the controlled substance administrations selected: (1) were not available for review due to missing MARs, (2) were not signed off by the person who administered the medication, (3) included the wrong dosage, or (4) did not include a prescription for the medication administered.
We also found that quarterly inventories submitted by BOP pharmacists to BOP headquarters did not always include all controlled substances. Specifically, we identified controlled substances at three of the institutions that should have been included with the mainstock in the quarterly inventory. We also found that 10 out of 12 institutions audited did not include controlled substances substock in their quarterly inventories. Pursuant to BOP policy, the institutions are only required to include mainstock in the quarterly controlled substances inventories; however, federal regulations require all controlled substances be included in the inventories required by the Drug Enforcement Administration (DEA). Further, given the numerous recordkeeping errors related to controlled substances noted in this report, in our judgment it is important that a complete accounting of all controlled substances is conducted on a quarterly basis.
We identified inadequate internal controls related to purchasing of prescription medications, including ordering, receiving, and payment. At each institution audited, we did not find any evidence of segregation of duties related to purchasing of prescription medications. At most institutions the person who ordered the prescription medications was the same person who received and inventoried the shipment, and signed off on the invoice before it was submitted to the business office for payment. The BOP currently does not have a national policy related to internal controls over the purchasing of prescription medications, and relies on each institution to develop and implement its own policies and procedures. The lack of internal controls resulted in a Chief Pharmacist being able to fraudulently purchase 30,600 doses of prescription medications between July 2002 and February 2004 for his personal consumption.7 This cost the BOP approximately $1,567, with a retail value of approximately $28,700.
Pharmacy Compliance
We found that the BOP pharmacies were not always in compliance with applicable BOP policies and procedures regarding the dispensing and administering of prescription medications. At the 12 BOP institutions included in our audit, we reviewed 1,107 prescriptions, including 488 prescriptions for controlled substances, and found that 384 (35 percent) of the prescriptions reviewed were not in compliance with BOP policy. Specifically, we found:
- 206 prescriptions for which the pharmacist's review for contraindications was not documented,
- 54 controlled substance prescriptions for which the prescription forms were missing a DEA registration number or required signature,
- 31 prescriptions for non-formulary medications for which the required waiver was not obtained8,
- 24 controlled substances prescriptions for which the required separate written prescription forms were not maintained by the institution,
- 20 controlled substances prescriptions that were written for longer than the allowable time period,
- 19 prescriptions for which required information was missing in the inmate's medical file, and
- 30 prescriptions with other miscellaneous errors.
Recommendations
Our report contains 13 recommendations for the BOP to improve the administration of its Pharmacy Services. Specifically, our recommendations seek to ensure that:
- an adequate cost-benefit analysis is conducted for all cost savings initiatives prior to implementation and that the initiatives are implemented consistently throughout all institutions;
- institutions accurately account for and safeguard their prescription medications, especially controlled substances;
- institutions implement controls over ordering and receiving prescription medications that provide for adequate separation of duties; and
- institutions comply with applicable laws and BOP policies.
- Department of Heath and Human Services, National Center for Health Statistics, Health, United States, 2004.
- Due to the availability of data, the data for the Change in Consumer Prescription Drug Costs is based on the calendar year, while the Change in the BOP Inmate Population and the Change in the BOP Prescription Drug Costs are based on fiscal year.
- Contraindications include drug to drug, drug to disease, and drug to food interactions; therapeutic duplications; allergies; therapeutic inappropriateness; inappropriate doses; incorrect duration of therapy; and adverse drug reactions.
- Mainstock consist of the bulk inventory of controlled substances. The mainstock inventory is used to account for all purchases, disposals, and transfer to substocks. The substock consists of a smaller number of controlled substances dispensed from the mainstock and is used to administer medications to inmates on a daily basis.
- At the Springfield Medical Center, we judgmentally selected a sample of nine controlled substances, and only reviewed a 7-month period because of the large volume of use at the institution.
- Proof of Use sheets are logs that track substock inventory and include an inmate's name and number, quantity issued, date and time, and person administering the medication. MARs are individual inmate records used to track the receipt of medication and include an inmate's name and number, prescription medication name, strength, quantity, date, time, and person who administered the prescription medication.
- In lieu of prosecution, the Western District of Oklahoma offered the pharmacist a 1-year Pretrial Diversion Program and if all conditions are met, the pharmacist will not be prosecuted.
- The BOP National Formulary is a list of all prescription medications recommended as essential for inmate care and is used to help provide clinically appropriate, safe, and cost-effective prescription medications. If a non-formulary drug is deemed necessary, the prescriber is required to obtain a Non-Formulary Drug Authorization requesting approval for the use of non-formulary medication to treat a specific inmate need.