History and Mission
The Drug Enforcement Administration's (DEA) laboratory system was established in 1973, when the functions of several federal agencies related to controlling illegal drugs were combined to create the DEA. Forensic resources that had served the former Bureau of Narcotics and Dangerous Drugs became the basis for the DEA laboratories. Over time, the laboratory system's duties expanded to include forensic services to other federal law enforcement agencies, such as the Federal Bureau of Investigation (FBI) and the U.S. Customs Service, and other responsibilities such as assisting other nations evaluate their forensic facilities.
The DEA's laboratory system directly supports the DEA's overall mission. The laboratory system's mission statement states:
The mission of DEA's laboratory system is to provide analytical, intelligence, scientific and other forensic and administrative support, to Special Agents of the DEA, other federal law enforcement officers, and to the criminal justice system at large in order to assist with the enforcement of controlled substance laws and regulations of the United States.
The laboratory system supports DEA programs carried out by DEA headquarters, 21 domestic divisions, and 80 international offices. Those programs include investigating and preparing for prosecution major interstate, gang-related, and international violations; developing and disseminating drug-related intelligence; coordinating with state, local, and foreign law enforcement; reducing the demand for drugs; and providing training for state, local, and foreign law enforcement officials.
The DEA is operated pursuant to the Controlled Substances Act and the Code of Federal Regulations (CFR), Title 21, Food and Drugs, Chapter II - Drug Enforcement Administration, Part 1300. The Act and regulation, however, contain no guidance directing the operation of the laboratory system.
The CFR classifies the controlled substances tested by the laboratories. Schedule I substances are defined as substances with a high potential for abuse, no currently accepted medical use in treatment in the United States, and a lack of accepted safety for use under medical supervision. Examples of Schedule I substances are heroin, LSD, and marijuana. Schedule II substances are substances with a high potential for abuse, but with a currently accepted medical use in treatment in the United States, and with tendencies for abuse that may lead to severe psychological or physical dependence. Examples of Schedule II substances include cocaine, morphine, and methamphetamine.
The Office of Forensic Sciences
The DEA's Office of Forensic Sciences leads and oversees the laboratory system by performing strategic planning, obtaining resources, ensuring the quality of laboratory results, promoting research and staff development, developing training programs, and managing the forensic laboratories. The Office of Forensic Sciences coordinates with other DEA divisions on policies, operations, information systems, facilities, inspections, and investigations.
The Office of Forensic Sciences also coordinates with law enforcement agencies and professional organizations to improve services, share information and methods, establish standards, and promote improvement. These agencies and organizations include the Department of Homeland Security, the Office of National Drug Control Policy (ONDCP), the American Society of Crime Laboratory Directors / Laboratory Accreditation Board (ASCLD/LAB), and several scientific working groups.
The Office of Forensic Sciences co-sponsors and provides technical oversight to the Scientific Working Group for the Analysis of Seized Drugs (SWGDRUG).5 The SWGDRUG is an international working group created in 1997 to recommend minimum standards for the forensic examination of seized drugs and to seek international acceptance of the standards. The Office of Forensic Sciences also participates in the Scientific Working Groups on Friction Ridge Analysis, Imaging Technologies, and Digital Evidence.
A Deputy Assistant Administrator heads up the Office of Forensic Sciences. Two Associate Deputy Assistant Administrators, three Section Chiefs, and a Quality Assurance Manager report to the Deputy Assistant Administrator. Each of the laboratories is headed by a Laboratory Director, who reports to one of the two Associate Deputy Assistant Administrators.
Laboratories and Services
The DEA operates nine laboratories to perform forensic services and research. Seven regional laboratories located throughout the U.S. work with evidence submitted by the DEA's domestic field divisions and other domestic law enforcement agencies.6 Two additional laboratories, the Special Testing and Research Laboratory (STRL) and the Digital Evidence Laboratory (DEL), both located near the District of Columbia (in Sterling and Lorton, Virginia), address the following specialized needs:
- The STRL performs research, analyzes controlled substances for intelligence use, analyzes controlled substances from international sources, and analyzes toolmarks and logos on drugs and drug packaging. Work performed for intelligence purposes generates strategic intelligence through the analysis of drug samples obtained in the U.S. and foreign countries. The STRL's drug signature programs analyze samples to determine processing methods, geographic origins, and manufacturers for heroin, cocaine, and methamphetamine. The STRL also operates the Source Determination Program, which analyzes toolmarks and logos on tablets, capsules, LSD blotter paper, and cocaine, heroin, and steroid packaging to determine manufacturing sources.
- The DEL was created as a separate laboratory in March 2003 from a unit that began in 1994 as the Computer Forensics Program, under the STRL. The DEL retrieves digital information from electronic devices and media, such as hard drives, compact and floppy disks, data tapes and personal digital assistants, for investigative and intelligence purposes. Such services are provided only by this laboratory.
The seven regional laboratories and their locations are listed below. Two satellite laboratories, located in Kansas City, MO, and San Juan, PR, supplement the regional laboratories. The satellite laboratories are housed in space in non-DEA facilities and are staffed by forensic chemists who are employed and supervised by DEA staff. One mobile laboratory, located in El Paso, TX, is staffed on a rotating basis by regional DEA laboratory personnel.7
|
||||||||||||||
| Source: DEA, Office of Forensic Sciences. |
The facilities in New York City, Miami, Chicago, and San Francisco occupy space in office buildings. The STRL, Mid-Atlantic, South Central, and Southwest Laboratories are housed in new stand-alone buildings. The DEL resides in a new building that also houses the DEA's Office of Investigative Technology.
Regional laboratories analyze evidence, submitted by DEA field offices and other law enforcement agencies, for controlled substances and latent prints. Laboratory personnel also provide expert testimony in court and technical advice and support to law enforcement agencies at seized clandestine laboratories and other crime scenes. Regional laboratories employ forensic chemists and fingerprint specialists to perform these services.
Forensic chemists analyze exhibits to identify substances and other properties of the exhibits.8 When asked by law enforcement officials to participate at a clandestine laboratory scene, forensic chemists are responsible for ensuring that any chemical reactions in progress are safely shut down.
The DEA's latent print program began in the late 1970's with four positions spread over three laboratories to supplement services that were provided by the Federal Bureau of Investigation (FBI). The DEA expanded the latent print program in 1991 to six laboratories to improve the timeliness and responsiveness of latent print services to its field offices.9 Fingerprint specialists examine exhibits to develop and identify latent prints. They may be asked to test exhibits at a crime scene for fingerprints before others handle the evidence. They also develop photographic film for DEA field offices, and may be asked to photograph crime scene evidence.
In addition to these forensic analysis responsibilities, DEA laboratories maintain custody of all drug evidence seized by DEA field offices once it has been submitted for analysis, with the exception of bulk marijuana and bulk ephedrine.10 The laboratories receive, secure, account for, and eventually dispose of all DEA evidence exhibits submitted for drug analysis. The laboratories employ evidence technicians and scientific intelligence technicians to help maintain accountability over evidence.
Customers
The DEA field offices submit all DEA drug evidence, and some non-drug evidence on which latent print analysis is being requested, to the DEA laboratories.11 The DEA field offices also submit many exhibits that have been seized in joint or cooperative efforts with state and local law enforcement agencies. The DEA field offices also may request laboratory support at crime scenes.
Other federal customers of the DEA laboratories include the FBI; the Bureau of Immigration and Customs Enforcement (BICE); the Bureau of Alcohol, Tobacco, Firearms, and Explosives (ATF); the U.S. Park Police; the U.S. Coast Guard; and various agencies of the Department of Defense.12 In addition to submitting drug evidence for analysis to the regional laboratories, the two Customs agencies, now part of the Department of Homeland Security (DHS), also request latent print examinations. Federal agencies have also requested and received crime scene assistance from laboratory personnel. State and local agencies, such as the Metropolitan Police of the District of Columbia (MPDC) and the Kansas Bureau of Investigation, also submit drug evidence for analysis.
The chart below shows the percentages of exhibits submitted to the regional and digital laboratories, by customer, averaged for FYs 2000 through 2002.13 Exhibits submitted by DEA offices, including cooperative and joint seizures with other agencies, have consistently represented about two-thirds of the laboratories' work. All other federal agencies combined represented about another 24 percent, for nearly 90 percent of exhibits coming from federal agencies. State and local customers accounted for about 12 percent of all exhibits submitted for the 3 years, most of which were submitted by the MPDC.
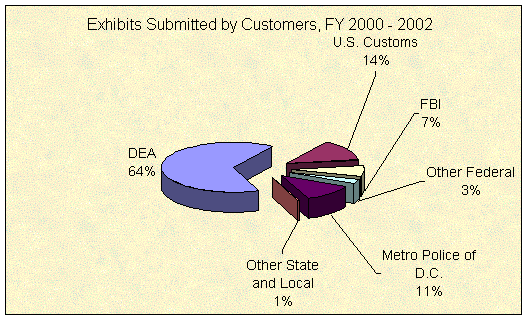 Source: DEA, Office of Forensic Sciences, System to Retrieve Information on Drug Evidence (STRIDE).14 |
Unlike most state and local law enforcement agencies, the MPDC does not perform its own forensic testing of drug exhibits and does not send exhibits to a state laboratory. The DEA's Mid-Atlantic Laboratory performs drug testing for the MPDC at no cost to the MPDC. The DEA has studied alternatives to this arrangement, but previously concluded that it is best to continue to provide these services directly to the District of Columbia.15
Transaction Volumes and Staffing
The regional and digital evidence laboratories received over 200,000 exhibits from FY 2000 through FY 2002.16 These were primarily domestic exhibits submitted to support investigations. The Mid-Atlantic and South Central Laboratories received the largest numbers of exhibits. The Mid-Atlantic Laboratory received about half of its exhibits from the MPDC.
|
||||||||||||||||||||||||||||||||||||||||
| Source: DEA, Office of Forensic Sciences, STRIDE.17 |
The table below reflects the number and types of services performed by the laboratories on exhibits during the past three fiscal years. Exhibits that were submitted for both drug and fingerprint analysis are counted twice in the figures below. This is one reason the number of exhibits analyzed each FY, shown in the table below, exceeded the number of exhibits received. Another reason is that the numbers below include the exhibits analyzed during FY 2002 that were received during the prior fiscal year.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Source: DEA Laboratory System Annual Reports.18 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The laboratories also perform other services, such as providing training for law enforcement officers, providing controlled substances to law enforcement for canine training, developing film, testifying, and assisting at clandestine laboratory seizures. From FY 2000 through 2002, the laboratories processed 765,466 photographic items (rolls of film, slides, and enlargements), provided expert testimony at 2,156 court appearances, and provided assistance at 568 clandestine laboratory seizures.
The number of exhibits received by all laboratories increased about 36 percent between 1996 and 2002, with increases in fingerprint and digital exhibits of about 52 percent and 198 percent, respectively. The increase in drug exhibits was about 34 percent. Expenditures for the Office of Forensic Sciences have grown from about $36 million in FY 2000 to almost $51 million in FY 2002, or almost 40 percent, and almost $60 million for FY 2003.
Laboratory staffing allocations for laboratory and support groups, not including supervisors, headquarters staff, or the STRL, increased since our prior audit from 219 positions in FY 1993 to 318 allocated positions for FY 2003, an increase of about 45 percent. Staffing levels for all laboratory positions, by laboratory, allocated for FY 2003 (including supervisors and the STRL) are shown in the table below.
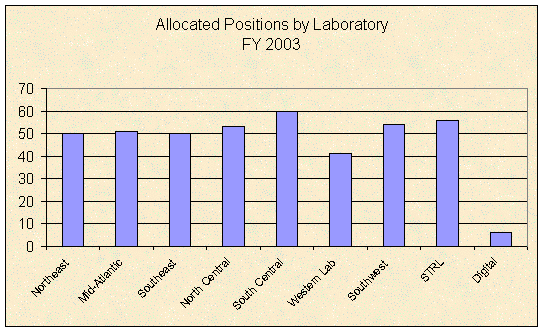 Source: DEA, Office of Forensic Sciences. |
Information Systems
The DEA laboratories use two automated information systems to record and retrieve information about evidence.19 The System to Retrieve Information on Drug Evidence (STRIDE) is an older DEA database originally designed to record inventory and analysis information on drug exhibits. As DEA laboratories expanded into the additional disciplines of fingerprint and digital examinations, the DEA modified the STRIDE to record some data about these exhibits.20 The STRIDE currently contains complete analysis data only for the analysis of controlled substances. It does not contain analysis information related to fingerprint examinations on drug exhibits, any results of latent print analyses, or any analysis information about digital evidence exhibits. During our audit, the STRIDE also did not contain inventory information on fingerprint-only exhibits in one laboratory. The STRIDE includes functionality for tracking exhibits from receipt through destruction. Scientific intelligence technicians enter most data into the STRIDE.
The Laboratory Evidence Management System (LEMS) is a DEA database designed to track the movement of exhibits within laboratories, when they are transferred out to agents or defense attorneys for court purposes, and when they are destroyed. The LEMS creates and reads bar codes associated with exhibits, and produces a variety of reports to support complete physical evidence inventories, destruction, and other activities. Evidence technicians enter data into the LEMS and scan bar-coded exhibits after they are labeled; analysts scan coded identification cards into the LEMS to track the movement of exhibits within the laboratories.
The STRIDE was used as the central repository of information about exhibits, and the LEMS was used to track the movement of exhibits in and out of individual vaults and laboratories.
Laboratory staff also entered inventory information about exhibits into various manual logs and other records to help track evidence and actions. Laboratories performed duplicate and inefficient data entry to keep the various systems of records updated. The LEMS was intended to replace manual recording on DEA-307 index cards, which serves the same functions.21 However, the laboratories continue to maintain DEA-307 cards to use for back up when the LEMS goes down, and because LEMS did not always function as intended when it was implemented.
The DEA initiated a contract in June 2003 to integrate its automated and manual information systems and improve functional support to its laboratories. As part of this integration project, the DEA plans to implement radio frequency tags and scanners that should simplify accountability over exhibits. The system should also make accountability far more efficient than was the case during our audit. The system is scheduled to be pilot tested in the summer of 2004.
Laboratory System Strategic Plan
The DEA Laboratory System Strategic Plan for FY 2002 - 2007 presents the DEA's assessment of its laboratory system and strategies for improvement. The DEA obtained input from customers, consultants, and laboratory managers and employees to help identify problems and develop an action plan for improvement. The Plan calls for increased specialization of supervisors over fingerprint services and vaults, increased staffing to improve turnaround times to customers, and technological improvements for matching latent prints.
Prior Audits
The OIG published Audit Report 95-18, Drug Enforcement Administration's Laboratory Operations, in May 1995. This audit reported weaknesses in laboratory facilities but found that DEA laboratory operations and management controls were generally satisfactory. Customer satisfaction ranged from favorable to excellent. The audit contained several findings regarding facilities and controls over evidence:
- Several laboratory facilities were outmoded and overcrowded, although satisfactory for laboratory personnel to perform essential duties.
- Customers reported that laboratories provided timely assistance to their investigations, but several commented that faster turnaround of results would be helpful.
- Controls over exhibits and other controlled substances were satisfactory.
The prior audit recommended that the DEA enhance controls over evidence to ensure compliance with DEA policies on recording weights of exhibits after analysis, and to reduce the time between completing analyses and returning exhibits to vaults. The DEA responded by establishing policies to address these recommendations and we found no material weaknesses in these functions. The prior audit recommended that the DEA consider consolidating its laboratories if it was unable to obtain funding to replace the outmoded facilities. The prior audit also recommended that the DEA reduce the Mid-Atlantic Laboratory's workload by seeking alternate providers for serving the MPDC. In response, the DEA did not concur with the latter two recommendations. The DEA was able to obtain the funding needed to replace regional facilities and continues to provide services to the MPDC; therefore, no changes were made to these practices.
Earlier Department of Justice audits also found varying levels of compliance with controls over evidence. Audit Report 87-14-SI, Drug Enforcement Administration's Internal Controls over Seized Evidence, April 1987, found that the DEA's system of internal controls promoted accurate accounting and adequate safeguarding of seized evidence by the division offices and laboratories, although it allowed instances of non-compliance with procedures. Audit Report 89-6, The Department of Justice's Internal Controls Over Seized Evidence, December 1988, recommended the Office of Forensic Sciences ensure that physical inventories are performed at prescribed intervals by persons independent of evidence custodial functions.
Footnotes
- The co-sponsor with the DEA is the Office of National Drug Control Policy.
- Each domestic field division supervises several district and resident offices. The Caribbean Field Division in San Juan, PR, is classified as a domestic division. Several international offices in Caribbean nations, such as Jamaica and Haiti, are affiliated with this field division.
- The mobile laboratory was deployed to Tucson, AZ in FY 2001 and was later moved to El Paso.
- In addition to suspected substances, exhibits may include items such as apparently empty containers to be tested for trace amounts of drugs.
- DEA officials reported that the DEA was not satisfied with the slow turnaround and lack of priority from the FBI laboratory on latent prints. The DEA officials indicated the lack of priority was understandable because of competing high-priority demands at the FBI and the fact that expectations for developing usable latent prints are low for drug packaging.
- The field offices prepare samples of bulk marijuana and ephedrine seizures to send to laboratories for testing, and maintain custody of the bulk quantities. The field offices also maintain custody of DEA non-drug exhibits. Non-drug exhibits submitted to laboratories for fingerprint analysis are returned to the field offices after analysis.
- The DEA considers all evidence submitted for drug analysis as drug evidence and exhibits submitted for latent print or digital analysis only as non-drug evidence.
- The former U.S. Customs Service was re-organized in March 2003 into two separate agencies in the Department of Homeland Security: BICE and Customs and Border Protection. During the audit period these agencies were called the U.S. Customs Service.
- The STRL is not included because most of the exhibits it receives to analyze for intelligence purposes are samples of exhibits that were originally sent to the regional laboratories by the customers represented in the chart.
- The figures for DEA include exhibits submitted in joint and cooperative efforts between DEA and other law enforcement agencies. Figures for the FBI include exhibits submitted by the FBI and by joint FBI-Organized Crime Drug Enforcement Task Forces.
- DEA presented this conclusion in a decision paper dated December 9, 1994, based in part on the unique relationship between the MPDC, which enforces laws, and the U.S. Attorneys Office, which adjudicates cases for the District of Columbia.
- This figure counts each exhibit submitted for both drug and latent print analysis as one exhibit.
- The table does not include exhibits submitted to the STRL because most of those exhibits are samples of exhibits that were originally sent to the regional laboratories and are already represented in the table. The figures include those drug exhibits that are submitted for storage only and do not require analysis, as the laboratories serve as custodian for all DEA controlled substances other than bulk marijuana and bulk ephedrine. Such exhibits may be seized after being abandoned, with no case or suspect for the DEA to pursue. Others may be multiple exhibits from a seizure, not all of which are analyzed.
- The figures count exhibits submitted for both drug and fingerprint analysis twice, once for each type of analysis.
- Individual laboratories have created and maintain some additional information systems containing local data about exhibits.
- Technically the current system is STRIDE II, the enhanced version of the original STRIDE.
- The DEA-307, Evidence Accountability Record, is a 5" by 8" index card used to record manually the movement of exhibits that is also recorded in LEMS electronically.
*Because this report contained information designated as "Law Enforcement Sensitive" by the Drug Enforcement Administration, we redacted (whited out) that information from the version of the report that is being publicly released. Where such information was redacted is noted in the report.